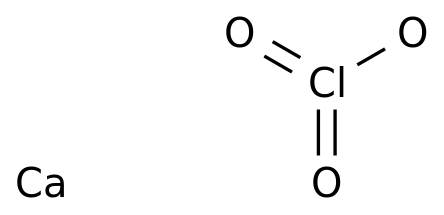
If liquid water exists on the surface of Mars, it is most likely in the form of a briny mixture with magnesium calcium chlorate salts, according to new experiments based on discoveries previously made by NASA’s Phoenix and Viking landers, as well as the Curiosity rover.
Gases, liquids and solids
The ‘triple point’ of a substance is the temperature and pressure at which it can co-exist in all three phases: gas, liquid and solid. For water, the triple point is found at 0.01 degrees Celsius (32 degrees Fahrenheit) and 6.12 millibar, or 0.6% of the atmospheric pressure at the Earth’s surface. In other words, one might imagine a bucket of water at the triple point, where the water exists as ice floating on a layer of liquid water, with water vapor just above the ice that has sublimated or evaporated from it. The vapor that is in contact with the ice exerts a pressure on the ice, which we call the vapor pressure.
In 2008, the Phoenix lander’s Thermal Evolved Gas Analyzer (TEGA), which was part of its on-board Wet Chemistry Lab, found calcium chlorate in soil samples from Mars’ north polar region, at concentrations of 0.4–0.6%. This encouraged scientists to re-analyze data from soil samples from the Viking lander missions, which took place in the 1970s.
The new analysis suggested that the soil found at Chryse and Utopia Planitiae by the Viking landers contained calcium chlorate at a concentration less than or equal to 0.1%. Then, in 2013, the Curiosity rover’s Sample Analysis at Mars (SAM)instrument found calcium chlorate in soil samples from Rocknest, which is a spot within Gale Crater.
Mixing salts
Which of these chlorates and perchlorates would be most likely to be dissolved in water in Martian conditions?
The University of Washington’s Jonathan Toner and David Catling had previously modeled data from Phoenix’s Wet Chemistry Lab, to understand how different salts behave in Mars’ freezing temperatures. They found that the soil samples likely containedmagnesium sulfate, magnesium chlorate, sodium chlorate, potassium chlorate, sodium chloride and calcium carbonate.
In theirlatest study, Toner and Catling made solutions from these salts. They found that, out of all the salt-water mixtures, the calcium chlorate solution had the lowest vapor pressure. This means that it is least likely to evaporate or freeze and is the most likely to absorb whatever low levels of moisture are present in the Martian atmosphere.
So to find liquid water on Mars, should scientists only look for sites on Mars that are rich in calcium chlorate?
“Any salts present in Mars soils will likely be as a salt mixture, so measuring the properties of these mixtures is important,” says Toner. Based on the soil chemistry measured by the Phoenix lander, Toner says that sodium and calcium chloratemixturesare most likely, whereas calcium chlorate mixtures are unlikely to be found.
Water for life
Could there be enough water in these brines to support microbial life? Studies of extremophiles grown in perchlorate and calcium chlorate solutions suggest that microbes could survive in brines that may exist on Mars. A group of scientists led by Mark Schneegurt, a professor of biological sciences at Wichita State University in Kansas, USA, found that several species of halotolerant, i.e. salt-tolerant bacteria, were able to grow in high concentrations of calcium chlorate salts.